Antibodies as Neurotransmitters: A Theory for the Understanding of the Complexity of Neuroimmune Crosstalk and Whole Body Homeostasis.
Introduction
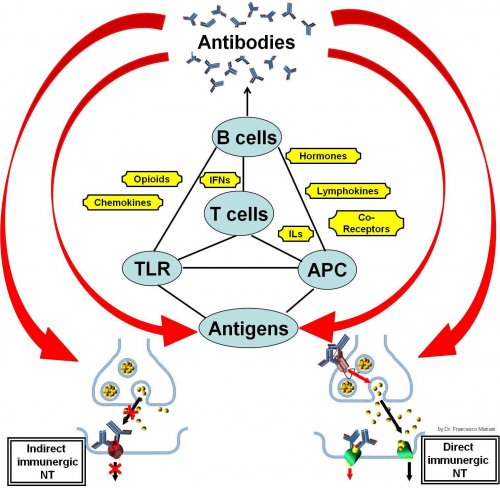
Since the Nobel prize winner Niels K. Jerne suggested the possibility that immune system is regulated by an intricate network of interacting molecules (idiotype network theory, 1976), immune system has not only been interpreted as the self/non-self recognizing apparatus, but also as a gateway by which homeostasis perturbation can result in immune reactivity or immune tolerance[1,2].
Immune system is regulated at many levels by interactions between hormones[3,4], brain peptides[5,6,7], cytokines[8,9], chemokines[10,11], vitamins[12-14], inflammatory mediators[15,16], and a great number of receptors (including Toll-like receptors[17,18] and others[19-22]). The complexity of this tight communication between systems influences different patterns of activation and response of our immune system. The levels of activity of these connections are reciprocal and multidirectional, as it has been documented in many research works since the late 1980s[23-25].
Today, studies addressed in elucidating which antibodies are directed to which antigens and the impact antibodies have in the development of different diseases are exponentially growing, with the implementation of new methods and techniques[26]. This approach is producing a plethora of new antibodies that researchers are trying to characterize in a statistical relationship with different clinical conditions. Anyway, we must admit that for a large part of cases the significance of the presence of circulating antibodies remains poorly understood. In many clinical or experimental situations, antibody production seems inevitably to remain an “epiphenomenon” (i.e. cross-reactivities without a clear role). For an overview of the problematics regarding circulating antibody significance we suggest a selection of the literature[27-39].
Our plasma cells produce a lot of antibodies and if the sense of antibody production were only the interaction with non-self, it would be excessively dispersive for the economy of the individuals and would not be in accordance with the growing evidence of an immune system subjected to genetic and evolutionally selected pattern of antibody reactivity[40-43]. Only in few cases, presence of autoantibodies may predict the development of pathological conditions[29-31] or its course in the years[32,33,36]. On the other side, circulating antibody significance and role are yet showing contrasting evidences[27,34,35,37]. The presence and permanence of myriads of different antibodies following infectious diseases, vaccinations, cancers, pollutant exposures, etc... or even in apparently healthy subjects remains a fascinating conundrum[38,39,44-49]. Finally, the production of antibodies in patients with autoimmune diseases treated with biological therapies (i.e. TNF-alpha blocker monoclonal antibodies, and others) is a paradoxical event that has not yet a clear explanation[50,51].